CHAPTER17
Solving Human Problems
BRAIN-MACHINE INTERFACE
 If you know someone with severe neurological damage — perhaps cerebral palsy, trauma from a car or motorcycle accident, a military or sports injury, or a stroke — you’ve seen, firsthand, how communicating with the outside world and performing daily tasks can be a challenge. But during the past few decades, scientists have made impressive progress in developing technologies that can bypass such damage. Now brain-machine interfaces can read the activity of millions of neurons — through electroencephalographic (EEG) activity from the brain’s surface or from implanted electrodes — and predict the behavioral intentions of research participants. These advances give human and animal subjects neural control of parts of their surroundings: from computer cursors to video games to robotic limbs.
If you know someone with severe neurological damage — perhaps cerebral palsy, trauma from a car or motorcycle accident, a military or sports injury, or a stroke — you’ve seen, firsthand, how communicating with the outside world and performing daily tasks can be a challenge. But during the past few decades, scientists have made impressive progress in developing technologies that can bypass such damage. Now brain-machine interfaces can read the activity of millions of neurons — through electroencephalographic (EEG) activity from the brain’s surface or from implanted electrodes — and predict the behavioral intentions of research participants. These advances give human and animal subjects neural control of parts of their surroundings: from computer cursors to video games to robotic limbs.
Despite the sci-fi sizzle, most of the work on electronic brain implants is derived from basic research on how animals and people plan and control various types of movement. Using hair-thin wires inserted into the brains of monkeys and rats, scientists first recorded the firing patterns of cells located in the premotor, primary motor, and posterior parietal cortical areas of the brain. As the animals performed repetitive tasks like pressing a lever to receive a reward, researchers found specific firing patterns associated with the motion. Eventually, these patterns were translated into computer algorithms that allowed animals to complete a task via a robotic arm or prosthetic device simply by thinking about it.
The clinical applications of brain-machine interfaces quickly became clear. Neuroscientists and surgeons implanted electrode arrays in the brains of patients with epilepsy, paralysis, stroke, or Lou Gehrig’s disease (ALS), in hopes of enabling them to communicate and, someday, move independently. In early experiments, patients were only able to gain rudimentary control of a computer cursor. But a breakthrough occurred in 2011 when, after months of extensive training, quadriplegic patients learned to control movements of a third (robotic) arm — enabling them to grasp a drink of water or reach out to a loved one.
Honing this technology is allowing patients to control their own paralyzed limbs. Electrode chips implanted in their brains are connected to sleeves or gloves worn over the injured limbs. Sending tiny blasts of electricity into the patient’s nerves, located under the sleeve or glove, can reanimate paralyzed muscles. But brain-machine interfaces won’t become part of clinical medicine until they’re simplified, miniaturized, and made more reliable. Devices that wirelessly transmit commands from brain implants are a step in that direction.
A parallel line of research has explored applying this technology in the broader field of neuroprostheses. Neuroprosthetic devices not only receive output commands from a patient’s nervous system, but can also provide input — as occurs in retinal implants and prosthetic limbs. Prosthetic arms, for example, have remained frustratingly low-tech, but some brain-guided prostheses have integrated nerves and muscles at several different levels, allowing users to perform more precise and natural movements, and even enabling some to “feel” again. Still, even the most sophisticated neuroprostheses (such as brain implants) are limited by their number of electrodes and the lifespan of the implanted electrodes. Current arrays can only connect to 100 or so neurons, so a more complex and useful bionic future is still far away. Yet scientists and entrepreneurs are already thinking of new uses for the technology: restoring memory; enhancing cognition; and treating diseases such as depression, Alzheimer’s, and epilepsy.
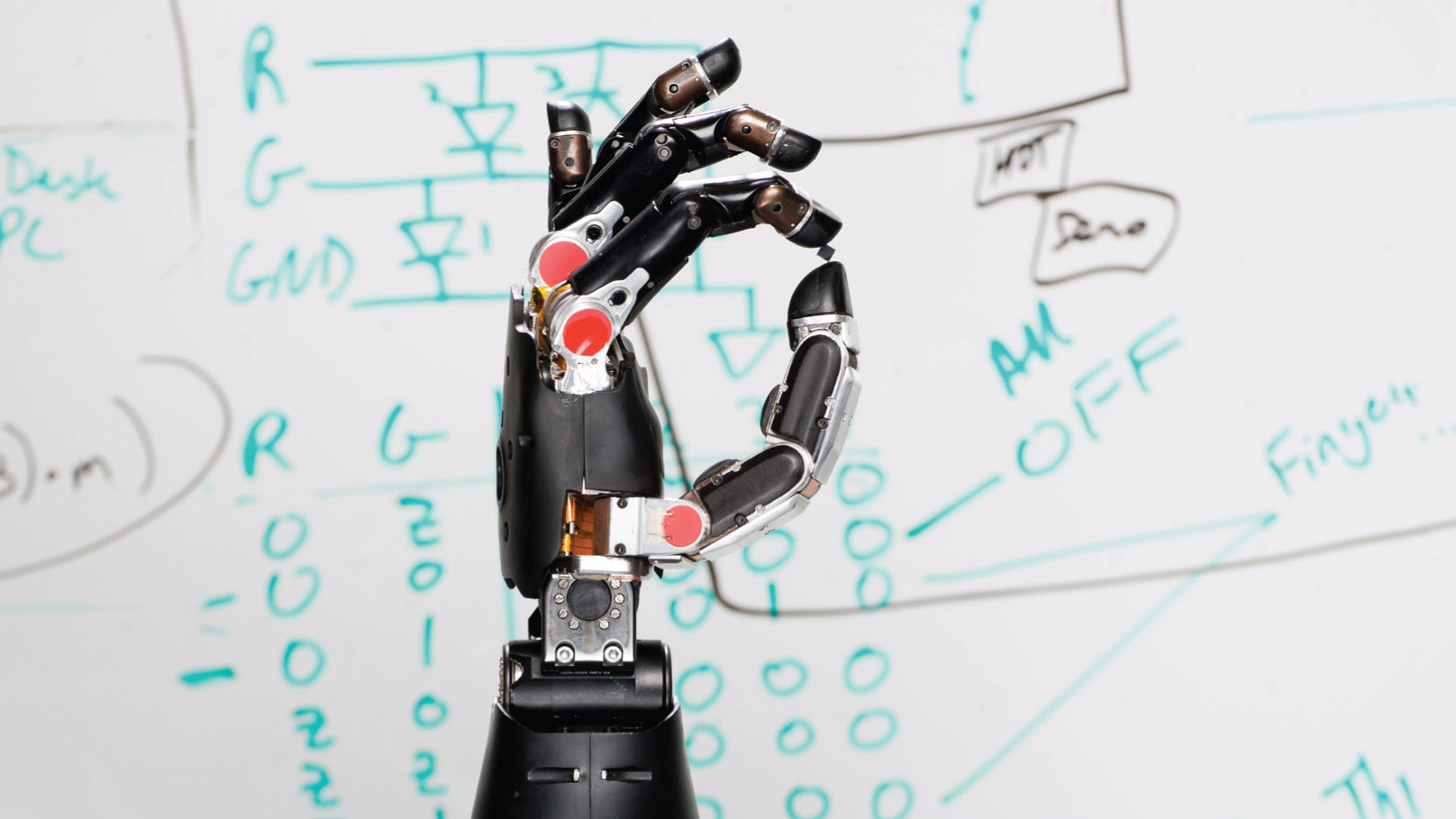
DARPA.
Advances in brain-machine interfaces are leading to incredible treatments, like this prosthetic arm that is controlled through thought.
DEEP BRAIN STIMULATION
 Insights into the pathophysiology of movement disorders have rekindled interest in the use of focused electrical stimulation as a form of treatment. The most advanced and precise method — deep brain stimulation (DBS) — was inspired by pacemakers engineered for the heart. Instead of electrodes implanted in the heart, the electrodes of the DBS device are surgically embedded in specific brain regions. Depending on where the electrodes are placed, DBS devices can help alleviate the symptoms of some brain disorders.
Insights into the pathophysiology of movement disorders have rekindled interest in the use of focused electrical stimulation as a form of treatment. The most advanced and precise method — deep brain stimulation (DBS) — was inspired by pacemakers engineered for the heart. Instead of electrodes implanted in the heart, the electrodes of the DBS device are surgically embedded in specific brain regions. Depending on where the electrodes are placed, DBS devices can help alleviate the symptoms of some brain disorders.
During most of these implantation surgeries, patients remain awake so that a neurologist can talk to them and ensure that the electrodes are stimulating the correct locations. While the patient’s head is held in place with a stereotactic frame, the surgeon drills a dime-sized hole (or smaller) in the skull. Then, a thin insulated wire with electrodes at the tip or along the shaft is inserted deep into the brain; if both sides of the brain are to receive implants, a wire is inserted into each side. In a separate surgery, a battery-operated pulse generator is implanted in the upper chest and connected to the electrodes. When the device is turned on, it starts sending electrical currents that alter the activity of the targeted brain cells.
The implanted device relies on the fact that neuronal communication uses electrical signals. In many movement disorders, an abnormal signal or pulse can gain control of a circuit and can easily become magnified. Like someone shouting in a crowded room, this aberrant signal can drown out other activity. DBS interrupts the shouting, so that normal communication can continue.
To determine where brain activity needs to be silenced or induced, neurosurgeons must identify the locations of the problems. The brain areas first targeted for tremors and Parkinson’s disease were chosen after years of painstaking neuroimaging, neuroanatomy, and fundamental research, especially in nonhuman primate models. Since then, deep brain stimulation has been used to treat epilepsy, dystonia, Tourette’s syndrome and, more recently, obsessive-compulsive disorder. Now researchers are investigating whether the DBS technique can potentially be extended to mood disorders such as treatment-resistant depression, as well as other complex mental disorders.
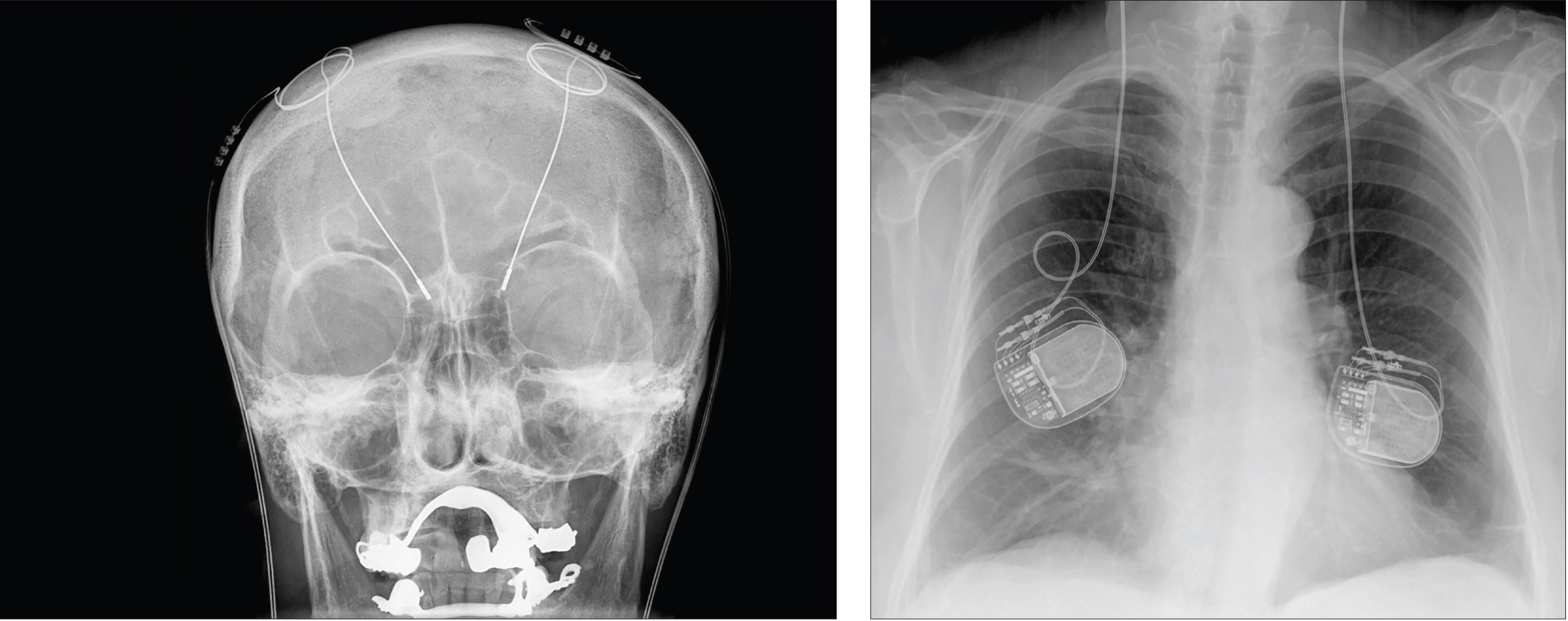
Hellerhoff.
Deep brain stimulation uses electrodes implanted deep in the brain, which carry electric impulses to specific brain regions. The power packs that provide the electricity are implanted in the patient’s back, as seen in this X-ray.
Yet DBS, like any surgical procedure, is not without some risks. It is highly invasive, and potential complications include infection, stroke, and bleeding in the brain. It also requires regular neurological follow-up and battery changes every 3 to 4 years.
PSYCHOACTIVE THERAPIES
Transcranial Stimulation
 A few noninvasive treatments can stimulate cells near the surface of the brain: Transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS) all use magnetic fields or low electrical currents to alter neural activity in a specific region of the human cortex and, indirectly, deeper brain structures to which it connects.
A few noninvasive treatments can stimulate cells near the surface of the brain: Transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS) all use magnetic fields or low electrical currents to alter neural activity in a specific region of the human cortex and, indirectly, deeper brain structures to which it connects.
During TMS therapy, patients sit in a chair while a nurse or technician places a magnetic stimulator against their head. The device painlessly delivers brief magnetic pulses to the brain, similar in strength to those generated by magnetic resonance imaging (MRI) devices, but highly targeted. For patients with depression, pulses are focused over their left prefrontal cortex. Here, they generate electrical currents among neurons which, over time, help lift the patient’s mood.
Similarly, tDCS uses one or two milliamperes of direct current to tune the brain. Although research into tDCS and its close cousin, tACS, is in its early stages, these techniques offer clear advantages over deep brain stimulation and even over TMS. Generally, patients report only a slight tingling or tapping feeling on their head as the therapy is administered. The devices used to administer these therapies are also cheaper, more portable, and lower-tech compared to TMS and DBS.
A number of studies have suggested that tDCS and tACS might be used to improve working memory as well as to relieve chronic pain and the symptoms of depression, fibromyalgia, schizophrenia, and other disorders. However, despite the substantial literature, meta-analyses have failed to conclusively prove any effects of transcranial electrical stimulation. Currently, there is no consensus among scientists on how these treatments might work, or even on the best way to position the stimulation devices.
New Types of Drugs
Most doctors still recommend medication as the first line of treatment for neurological and psychiatric disorders. The antidepressants, antipsychotics, and other mind-altering medications used today have been tested in extensive clinical trials and remain largely unchanged from their prototypes developed in the 1950s. Each of these classes of drugs is now filled with subsequent generations of closely related drugs designed to interact more selectively with their targets, producing better therapeutic effects and fewer side effects, in some cases. Nevertheless, these are just slightly improved copies of one another. Truly novel drug candidates are rare, and hard to develop.
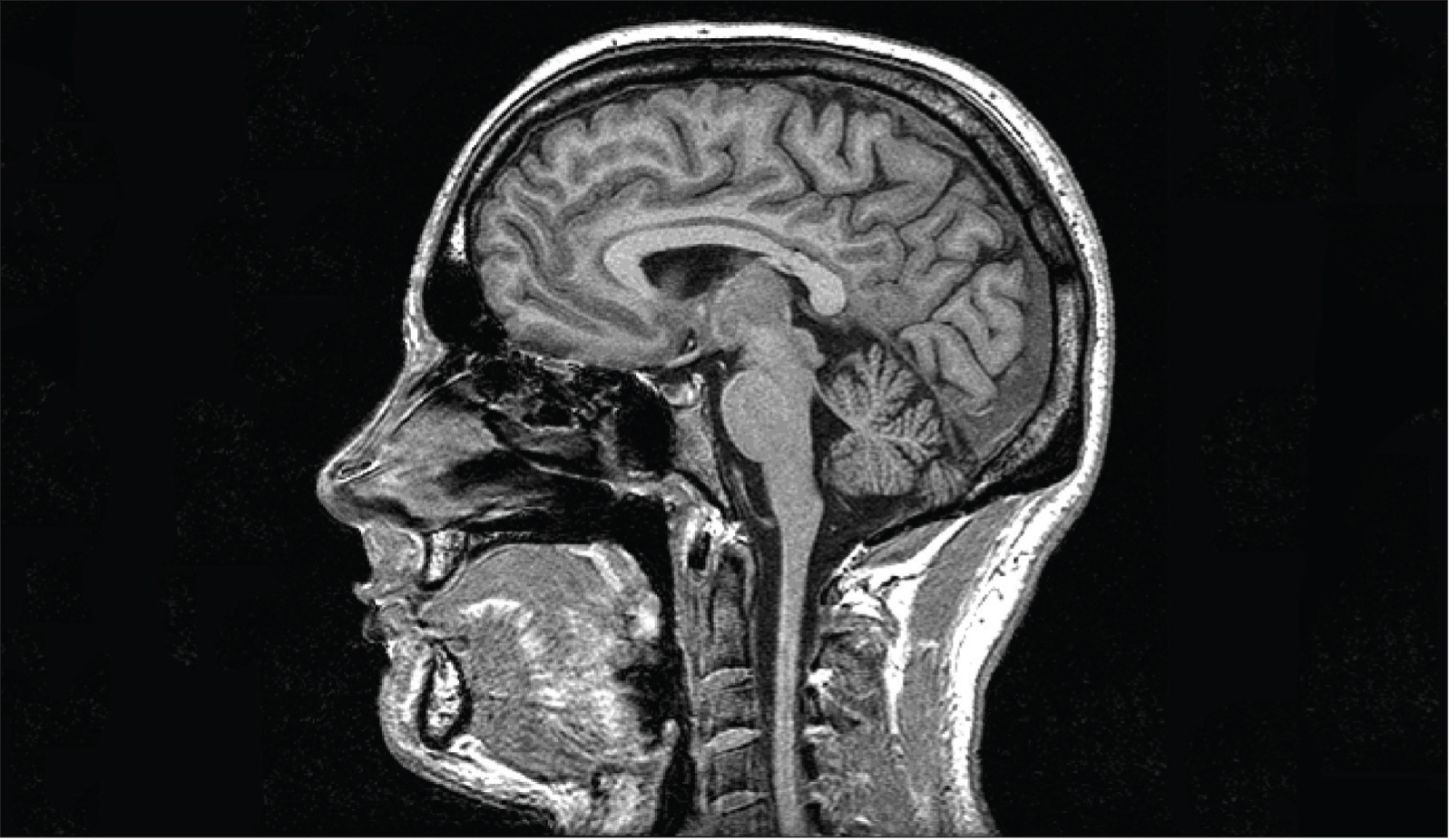
Oxford Centre for Functional MRI of the Brain, Oxford University.
MRI scans can provide detailed images of brain tissue. Here, it displays tissue high in water and fat content in white.
One of the biggest challenges in developing new drugs to treat neurological or psychiatric problems is finding molecules that can cross the protective blood-brain barrier — tightly packed endothelial cells lining blood vessels restrict the kinds of molecules that can enter the brain. While this barrier’s fortress-like quality is good for normal function, it prevents most drugs delivered by typical means — including pills, patches, injections, or enemas — from having any useful therapeutic effects within the brain. Scientists have had to design extremely tiny molecules or adopt ingenious strategies such as nanoparticles that shuttle in drugs, enzymes that activate molecules after they’ve “snuck through” the barrier, and antibodies that were specifically engineered for the brain.
Preliminary trials of drugs that use the body’s own immune system to confront and clear unwanted proteins from the brain have sparked a great deal of interest, particularly in the Alzheimer’s disease community. When mice and monkeys receive a vaccine that contains a major component of amyloid plaques, their immune systems develop antibodies capable of traveling to the brain and “tagging” the amyloid-beta plaques that are the hallmark of Alzheimer’s disease. This tagging seems to alert microglial cells in the brain, which head for the plaques and try to remove them. In some experiments, mice bred to develop an Alzheimer’s-like disease remembered how to navigate through a Morris water maze, after being vaccinated — indicating that such vaccines might also relieve symptoms of the disease.
In the past, however, many Alzheimer’s vaccines have failed in later-stage clinical trials. One reason for these failures was the development of harmful side effects. Several human participants experienced severe inflammation when their brains reacted to the antibodies against its proteins. Since then, newer approaches have engineered antibodies or antibody fragments that bind to their specific targets without triggering an autoimmune response. Other researchers have engineered double-duty antibodies. These use one end to sneak into the brain by binding to a receptor on the blood-brain barrier. Once inside, the antibody’s other end can cut off production of harmful amyloidbeta proteins even before plaques form.
By contrast, some therapies aim to boost helpful peptides and proteins called trophic factors, which are native to the brain. Neurotrophic factors support the growth and survival of specific groups of neurons. Scientists hope to modify these factors to reduce the amount of cell death in various neurodegenerative diseases.
The possible value of at least one trophic factor — nerve growth factor (NGF) — has already been demonstrated in several preclinical and early stage clinical trials. NGF slows the destruction of cholinergic neurons that plays a role in the cognitive decline of Alzheimer’s disease. Injecting NGF into patients’ brains stimulated the regeneration of these neurons and induced sprouting of new nerve fibers around the injection site. In some cases, evidence of this sprouting lasted up to 10 years after the initial therapy.
Brain-derived neurotrophic factor (BDNF) is showing potential for treating Alzheimer’s disease, as well as Huntington’s, Parkinson’s, ALS, and Rett syndrome. Moreover, the effects of boosting BDNF could even be stronger than those of NGF. But, in an interesting twist, the inhibition of some neurotrophic factors such as the neurite outgrowth inhibitor might also benefit patients. Studies have found that neurite outgrowth inhibitor is upregulated in the early stages of motor disease, and having too much of it around could prevent nerve regeneration. Scientists are now conducting clinical trials in which patients with ALS and spinal cord injuries receive custom-made antibodies to disable the neurite outgrowth inhibitor protein.
Ultimately, the ever-increasing global demand for therapies for neurological and mental diseases is a strong motivator for scientists and doctors in this field.
PREDICTIVE NEUROIMAGING AND PERSONALIZED MEDICINE
 As we gain understanding of the anatomical and functional changes underlying neurological illnesses, it becomes increasingly clear that these changes provide clues for earlier detection — even before symptoms appear. Many disorders, such as Alzheimer’s disease, are accompanied by specific brain activity and structural changes that can be tracked over time using MRI. By comparing this information with a baseline model of a healthy brain, researchers hope to predict which patients might one day develop neurological problems.
As we gain understanding of the anatomical and functional changes underlying neurological illnesses, it becomes increasingly clear that these changes provide clues for earlier detection — even before symptoms appear. Many disorders, such as Alzheimer’s disease, are accompanied by specific brain activity and structural changes that can be tracked over time using MRI. By comparing this information with a baseline model of a healthy brain, researchers hope to predict which patients might one day develop neurological problems.
Although it is still too early for these “markers” to be used as clinical reference points, they could pave the way for objective diagnoses of brain disorders, much as electrocardiograms and laboratory tests are currently used to reveal heart problems. The first step in this process is to produce a generic brain template by averaging the images from hundreds of randomly selected MRI scans. Scientists can then use machine-learning software to characterize the sets of healthy brain scans and the sets of scans known to show disease-associated changes.
Data from predictive neuroimaging can also be useful for guiding personalized treatment options and assessing a treatment’s clinical effectiveness. In studies of major depression, for example, patients whose brain scans showed an overactive amygdala (a brain region involved in emotional processing) were more likely to respond to psychotherapy. However, patients who exhibited higher activity in the anterior insula (another brain region involved in emotions) tended to improve with medication, but not with psychotherapy. In the future, psychiatrists could offer patients the best possible course of treatment based on their own biological characteristics, rather than relying only on symptoms or treatment preferences.
CELLULAR MARKERS
 In the past few years, a growing number of clinicians and scientists have rejected the boundaries of conventional DSM (Diagnostic and Statistical Manual of Mental Disorders)-defined diagnostic protocols that mental health professionals usually rely on. Rather than analyzing symptoms such as sadness, fatigue, or lack of sleep, the focus has shifted to finding biological markers that provide objective indices of those symptoms.
In the past few years, a growing number of clinicians and scientists have rejected the boundaries of conventional DSM (Diagnostic and Statistical Manual of Mental Disorders)-defined diagnostic protocols that mental health professionals usually rely on. Rather than analyzing symptoms such as sadness, fatigue, or lack of sleep, the focus has shifted to finding biological markers that provide objective indices of those symptoms.
Much like neuroimaging, cellular markers could be used to predict a patient’s risk and diagnosis before disease symptoms become obvious, as well as indicate how a patient may respond to certain treatments. The markers may be proteins, lipids, hormones, nucleic acids, or other compounds that can be detected in samples of blood, urine, saliva, or cerebrospinal fluid.
Although neuropsychiatric research on biomarkers still lags behind other fields such as oncology, researchers are investigating associations between genetic and cellular mechanisms and various mental disorders. A single biological cause for a mental disorder is hard to pin down — in fact, many skeptics say it is impossible to understand mental illness solely by understanding the brain. Causes of mental disorders are very complex and not easy to decipher. And yet, recent technological advances are enabling scientists to decipher more of the brain’s mysteries. Researchers can look deeper into the brain with imaging technology, map the circuits underlying specific mental states, and study how chemical levels change in individual neurons. Biomarkers reflect these physiological conditions, and studying them could lead to better targets for treatments. If chosen carefully, biomarkers might even provide useful ways to compare the effectiveness of treatments between patients, as well as in future clinical trials.
CELL TRANSPLANT
 To find new treatments for schizophrenia, stroke, Parkinson’s disease and other debilitating diseases, researchers around the world are turning to stem cells to study the biology of the diseases and disorders. These undifferentiated cells — from embryos or from certain adult tissues — have the remarkable potential to develop into any of the three major cell types of the brain: neurons; astrocytes, which nourish and protect neurons; and oligodendrocytes, which surround axons and enable them to conduct signals efficiently. Scientists hope that stem cells transplanted into the brain might be able to replace and repair neural cells that were lost due to disease or injury.
To find new treatments for schizophrenia, stroke, Parkinson’s disease and other debilitating diseases, researchers around the world are turning to stem cells to study the biology of the diseases and disorders. These undifferentiated cells — from embryos or from certain adult tissues — have the remarkable potential to develop into any of the three major cell types of the brain: neurons; astrocytes, which nourish and protect neurons; and oligodendrocytes, which surround axons and enable them to conduct signals efficiently. Scientists hope that stem cells transplanted into the brain might be able to replace and repair neural cells that were lost due to disease or injury.
In mice, stem cell therapy has reversed the signs of serious spinal cord injury. Within weeks of treatment, researchers observed that previously paralyzed mice could walk again. So far, only a few small trials of fetal and stem cell grafts have been conducted in humans. Some of the patients treated showed meaningful recovery from otherwise hard-to-treat disorders like stroke and Parkinson’s. Other trials were not successful, with replacement cells starting to produce excessive amounts of dopamine.
Thus, there are several challenges to overcome before successful use of neural stem cell transplant therapy. Embryonic cells and adult stem cells are difficult to harness and transplant into the brain. Controlling where and how stem cells differentiate into the necessary replacement cells is also tricky. Furthermore, stem cells carry a risk of being rejected by the recipient’s immune system. Scientists have recently discovered how to convert a patient’s own brain cells directly into dopamine neurons, which eliminates many risks, but the procedures are far from standard. None of them has yet been approved by the U.S. Food and Drug Administration.
GENE REPLACEMENT
 As researchers work to improve the safety and efficacy of genetic and cellular treatments, neuroscientists are finding new ways to deliver therapeutic genes into cells that need them. Designing therapies able to breach the blood-brain barrier is a challenge. Recent research has shown that small viruses with healthy genes tucked inside are able to cross the blood-brain barrier and replace faulty genes. Currently, adeno-associated virus and lentivirus seem to be the safest and most efficient vectors for gene therapy. These vectors are being used in clinical trials in patients with Parkinson’s and for some rare genetic diseases. Herpes simplex virus and adenovirus vectors have also been evaluated in early-stage human trials for treating brain tumors.
As researchers work to improve the safety and efficacy of genetic and cellular treatments, neuroscientists are finding new ways to deliver therapeutic genes into cells that need them. Designing therapies able to breach the blood-brain barrier is a challenge. Recent research has shown that small viruses with healthy genes tucked inside are able to cross the blood-brain barrier and replace faulty genes. Currently, adeno-associated virus and lentivirus seem to be the safest and most efficient vectors for gene therapy. These vectors are being used in clinical trials in patients with Parkinson’s and for some rare genetic diseases. Herpes simplex virus and adenovirus vectors have also been evaluated in early-stage human trials for treating brain tumors.
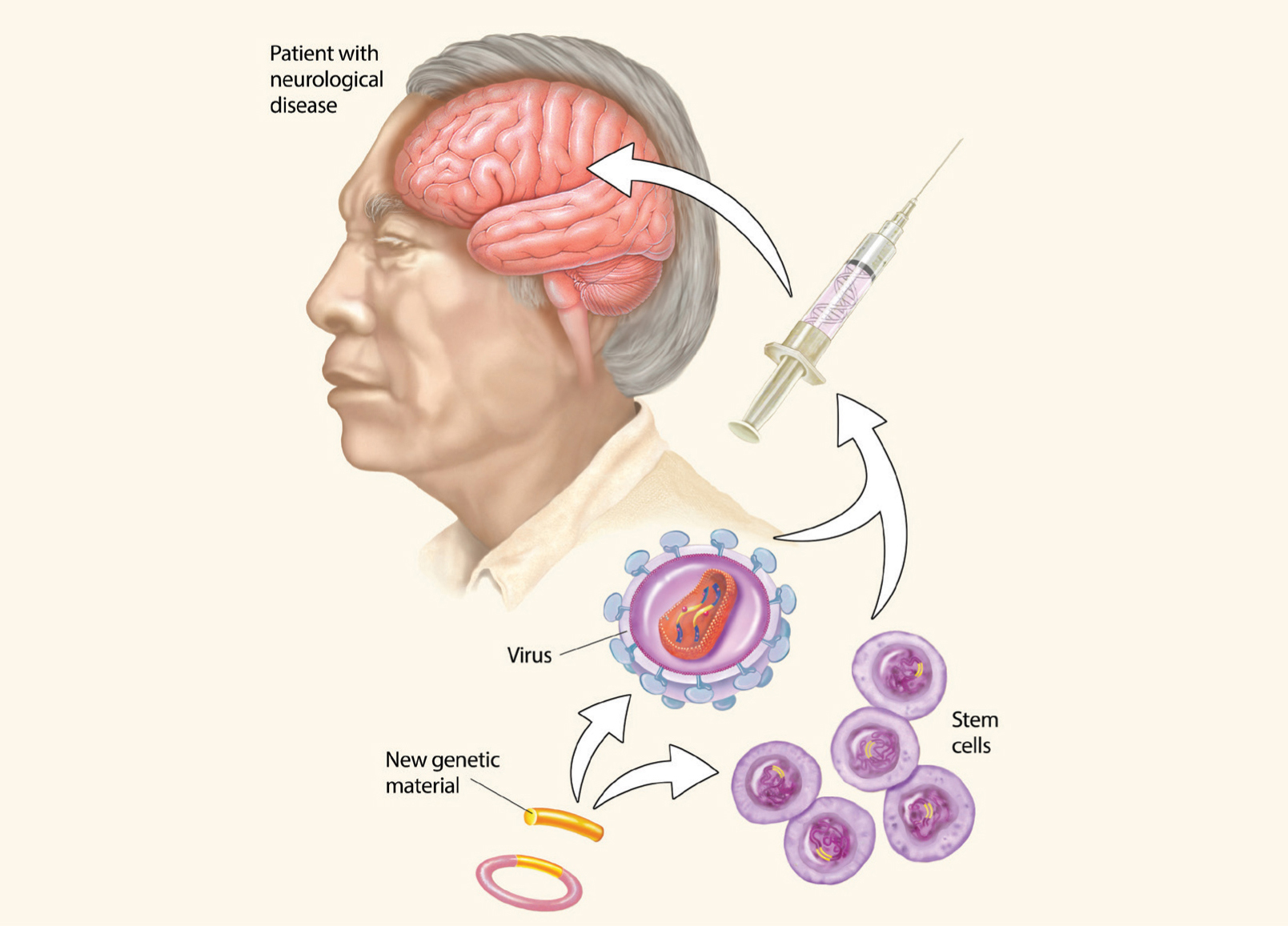
Stem cell and gene therapies hold huge potential for treating brain diseases. Therapeutic genetic material can be introduced in the brain though engineered viruses, while stem cells can be used to replace damaged or diseased cells in the brain.
In recent years, a new gene-editing method, CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), has begun to rewrite “conventional” gene therapy. The new technique uses RNA-guided enzymes to snip out or add DNA segments to a cell, allowing researchers to make extremely precise changes in a cell’s genome. Neuroscientists have already used CRISPR to repair part of a gene that produces toxic protein aggregates in the brains of mouse models of Huntington’s disease. When scientists looked at the mouse brains a few weeks after the procedure, the aggregated proteins typical of Huntington’s were almost gone, and the animals’ motor abilities had amazingly improved.
Of course, the usefulness of gene-editing technologies goes far beyond direct therapeutic applications. With CRISPR, dozens of mouse (and other animal) models can be made much more efficiently, facilitating studies of the brain and mental illness. But the technology is still relatively new, and it’s not perfect. The CRISPR system can make unintended cuts in the DNA if sequences are similar enough, so that unintended mutations could arise that affect the health of the animal being studied. In addition, this technology is not yet useful for treating complex conditions like schizophrenia and autism, which are thought to involve multiple genes. As with all new technologies, the ethical issues of using CRISPR as a gene therapy in humans are being hotly contested. Only time will tell whether CRISPR can be added to the expanding list of technologies that solve problems of the human brain. 
 If you know someone with severe neurological damage — perhaps cerebral palsy, trauma from a car or motorcycle accident, a military or sports injury, or a stroke — you’ve seen, firsthand, how communicating with the outside world and performing daily tasks can be a challenge. But during the past few decades, scientists have made impressive progress in developing technologies that can bypass such damage. Now brain-machine interfaces can read the activity of millions of neurons — through electroencephalographic (EEG) activity from the brain’s surface or from implanted electrodes — and predict the behavioral intentions of research participants. These advances give human and animal subjects neural control of parts of their surroundings: from computer cursors to video games to robotic limbs.
If you know someone with severe neurological damage — perhaps cerebral palsy, trauma from a car or motorcycle accident, a military or sports injury, or a stroke — you’ve seen, firsthand, how communicating with the outside world and performing daily tasks can be a challenge. But during the past few decades, scientists have made impressive progress in developing technologies that can bypass such damage. Now brain-machine interfaces can read the activity of millions of neurons — through electroencephalographic (EEG) activity from the brain’s surface or from implanted electrodes — and predict the behavioral intentions of research participants. These advances give human and animal subjects neural control of parts of their surroundings: from computer cursors to video games to robotic limbs.




