CHAPTER10
The Body in Balance
The cells of your body are immersed in a constantly changing environment. The nutrients that sustain them rise and fall with each meal. Gases, ions, and other solutes flow back and forth between your cells and blood. Chemicals bind to cells and trigger the building and release of proteins. Your cells digest food, get rid of wastes, build new tissues, and destroy old cells. Environmental changes, both internal and external, ripple through your body’s physiological systems. One of your brain’s less-visible jobs is to cope with all these changes, keep them within a normal range, and maintain the healthy functions of your body.
The tendency of your body’s tissues and organ systems to maintain a condition of balance or equilibrium is called homeostasis. Homeostasis depends on active regulation, with dynamic adjustments that keep the environment of your cells and tissues relatively constant. The brain is part of many homeostatic systems, providing signals that coordinate your body’s internal clocks and regulating hormone secretion by the endocrine system. These functions often involve a region of the forebrain called the hypothalamus.
CIRCADIAN RHYTHMS
 Almost every cell in your body has an internal clock that tells it when to become active, when to rest, and when to divide. These clocks broker changes in many of the body’s physiological systems over a 24-hour, or circadian, period. For example, the clocks cause faster pulses of peristaltic waves in your gut during the day and make your blood pressure dip at night. But because these clocks are deep inside your body and cannot detect daylight, none of them can tell time on its own. Instead, daily rhythms are coordinated by the suprachiasmatic nucleus (SCN), a tiny group of neurons in the hypothalamus.
Almost every cell in your body has an internal clock that tells it when to become active, when to rest, and when to divide. These clocks broker changes in many of the body’s physiological systems over a 24-hour, or circadian, period. For example, the clocks cause faster pulses of peristaltic waves in your gut during the day and make your blood pressure dip at night. But because these clocks are deep inside your body and cannot detect daylight, none of them can tell time on its own. Instead, daily rhythms are coordinated by the suprachiasmatic nucleus (SCN), a tiny group of neurons in the hypothalamus.
Neurons in the SCN act like a metronome for the rest of the body, emitting a steady stream of action potentials during the day and becoming quiet at night. The shift between active and silent states is controlled by cyclic interactions between two sets of proteins encoded by your body’s “clock” genes. Researchers first identified clock genes in the fruit fly Drosophila melanogaster and studied how they keep time; since then, a nearly identical set of genes has been found in mammals. The SCN also tracks what time it is based on signals it receives from photoreceptors in the retina, which keeps its activity in sync with the Earth’s actual day/night cycle. That little nudge is very important because, on their own, clock proteins take slightly more than 24 hours to complete a full cycle. Studies of animals deprived of light have discovered that they go to sleep and wake up a bit later each day.
An autonomic neural pathway ties the daily rhythmic activity of the SCN directly to other clocks in the body. Neurons in the SCN stimulate an adjacent region of the brain called the paraventricular nucleus (PVN), which in turn sends signals down a chain of neurons through the spinal cord to the peripheral organs of the body. You’ve already learned how signals in part of this neural pathway stimulate orexin neurons to regulate the body’s sleep/wake cycle. Related pathways also govern the secretion of melatonin, a hormone that influences sleep behaviors. Specifically, electrical activity originating in the SCN enters the PVN’s neural network and sends signals up to the pineal gland, a small pinecone-shaped gland embedded between the cerebral hemispheres. The pineal gland secretes melatonin into the bloodstream at night. Melatonin binds to cells in many tissues, and although it has no direct effect on clock gene expression in the SCN, its systemic effects seem to reduce alertness and increase sleepiness. Light exposure triggers signals that stop melatonin secretion, promoting wakeful behaviors.
Coordinated body clocks enable your body’s physiological systems to work together at the right times.
Together, these signals keep all the body’s clocks synchronized to the same 24-hour cycle. Coordinated body clocks enable your body’s physiological systems to work together at the right times. When your body prepares to wake from sleep, 1) levels of the stress hormone cortisol peak in the blood, releasing sugars from storage and increasing appetite, and 2) core body temperature begins to drift upwards, raising your body’s metabolic rate. These events, synchronized with others, prepare your body for a new day’s activity.
Desynchronizing the body’s physiological clocks can cause noticeable and sometimes serious health effects. You might have experienced a familiar example of circadian rhythm disturbance: jet lag. After crossing many time zones in a short time period, a person’s patterns of wakefulness and hunger are out of sync with day and night. Exposure to the local day/night cycle resets the brain and body, but it can take several days to get fully resynchronized. Circadian rhythms can also be disturbed by situations like late-shift jobs or blindness, which decouple normal daylight signals from wake/sleep cycles. Long-term circadian disruptions are associated with health problems including weight gain, increased rates of insomnia, depression, and cancers.
HORMONES, HOMEOSTASIS, AND BEHAVIOR
 Neurons can quickly deliver the brain’s messages to precise targets in the body. Hormones, on the other hand, deliver messages more slowly but can affect a larger set of tissues, producing large-scale changes in metabolism, growth, and behavior. The brain is one of the tissues that “listens” for hormonal signals — neurons throughout the brain are studded with hormone receptors — and the brain’s responses play an important part in regulating hormone secretion and changing behaviors to keep the body systems in equilibrium. The brain regions involved in hormone release are called the neuroendocrine system.
Neurons can quickly deliver the brain’s messages to precise targets in the body. Hormones, on the other hand, deliver messages more slowly but can affect a larger set of tissues, producing large-scale changes in metabolism, growth, and behavior. The brain is one of the tissues that “listens” for hormonal signals — neurons throughout the brain are studded with hormone receptors — and the brain’s responses play an important part in regulating hormone secretion and changing behaviors to keep the body systems in equilibrium. The brain regions involved in hormone release are called the neuroendocrine system.
The hypothalamus oversees the production and release of many hormones through its close ties to the pituitary gland. The paraventricular and supraoptic nuclei of the hypothalamus send axons into the posterior part of the pituitary gland; activation of specific neurons releases either vasopressin or oxytocin into capillaries within the pituitary. Both of these molecules act as neurotransmitters inside the brain, but they are also hormones that affect distant tissues of the body. Vasopressin (also called antidiuretic hormone) increases water retention in the kidneys and constricts blood vessels (vasoconstriction). Oxytocin promotes uterine contractions during labor and milk release during nursing.
Other hypothalamic regions send axons to a capillary-rich area above the pituitary called the median eminence. When these neurons are activated, they release their hormones into the blood. These releasing (and inhibiting) hormones travel through local blood vessels to the anterior pituitary, where they trigger (or inhibit) secretion of a second specific hormone. Of the seven anterior pituitary hormones, five are trophic hormones — these travel in the bloodstream to stimulate activity in specific endocrine glands (thyroid, adrenal cortex, ovaries, etc.) throughout the body. The remaining two hormones act on non-endocrine tissues. Growth hormone stimulates the growth of bone and soft tissues, and prolactin stimulates milk production by the breasts. Hormones released from the anterior pituitary influence growth, cellular metabolism, emotion, and the physiology of reproduction, hunger, thirst, and stress.
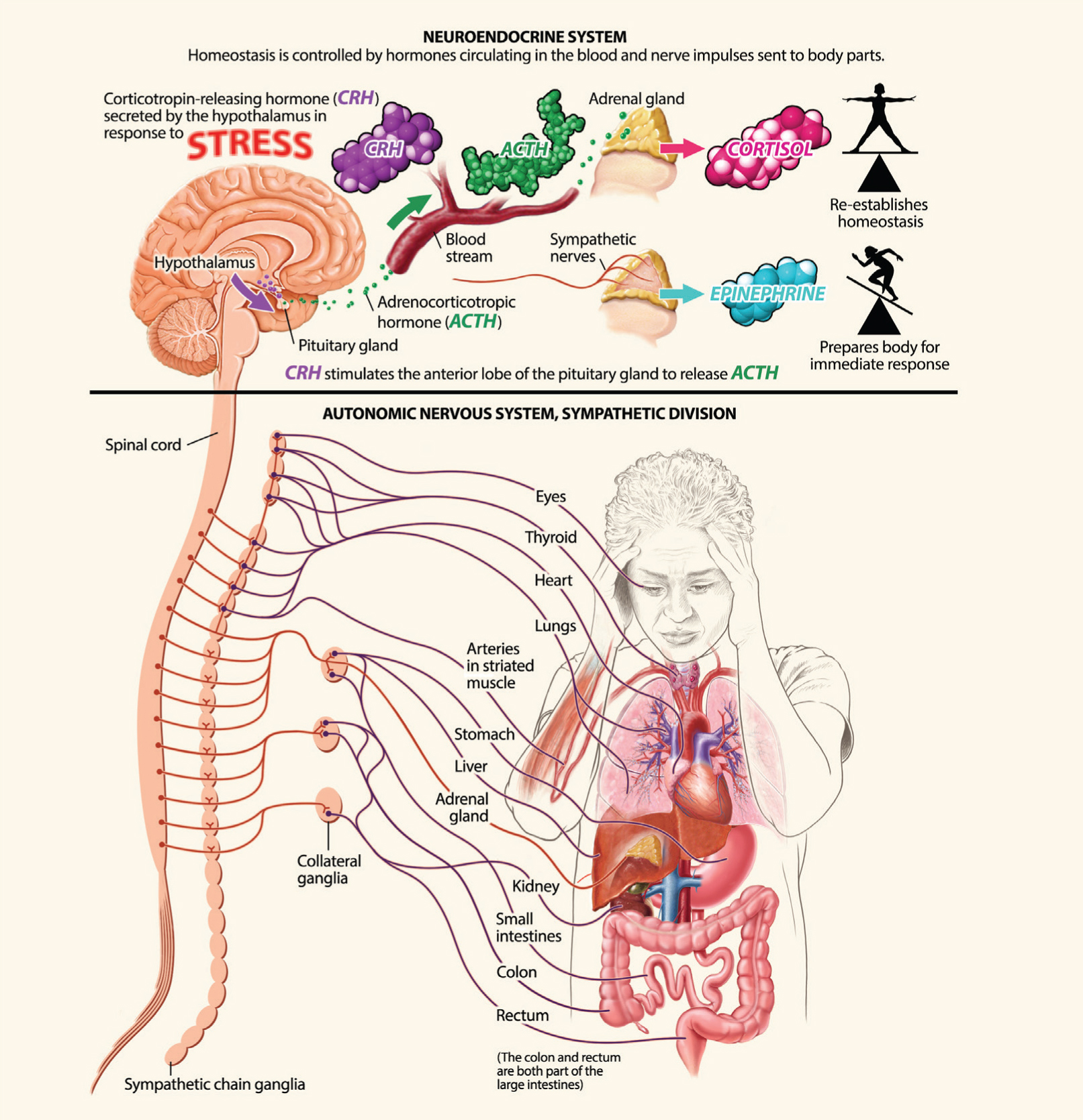
The neuroendocrine system maintains homeostasis, the body’s normal equilibrium, and controls the response to stress. The adrenal gland releases the stress hormones norepinephrine, epinephrine, and cortisol, which quicken heart rate and prepare muscles for action. Corticotrophin releasing hormone (CRH) is released from the hypothalamus and travels to the pituitary gland, where it triggers the release of adrenocorticotropic hormone (ACTH). ACTH travels in the blood to the adrenal glands, where it stimulates the release of cortisol.
Many hormones produced by the pituitary and its target endocrine glands affect receptors inside the brain — thus, these hormones can alter neuronal function and gene transcription in the hypothalamus. The effect is to reduce the amount of hormone released by the hypothalamus when those circuits become active. These negative feedback loops enable precise doses of hormones to be delivered to body tissues, and ensure that the hormone levels are narrowly regulated.
One of these three-hormone cascades regulates reproduction in mammals. Its underlying pattern is the same in both sexes: 1) gonadotropin-releasing hormone (GnRH) from the hypothalamus makes the anterior pituitary release 2) luteinizing hormone (LH) and follicle stimulating hormone (FSH), which in turn make the gonads secrete 3) sex hormones and start the development of mature eggs or sperm. Sex hormones, in turn, attach to receptors in the hypothalamus and anterior pituitary and modify the release of the hypothalamic and pituitary hormones. However, sex hormones regulate these feedback loops differently in males and females.
Male sex hormones induce simple negative feedback loops that reduce the secretion of gonadotropin-releasing hormone, luteinizing hormone, and follicle stimulating hormone. The interplay among these hormones creates a repetitive pulse of GnRH that peaks every 90 minutes. The waxing and waning of GnRH keeps testosterone levels relatively steady within body tissues, maintains male libido, and keeps the testes producing new sperm each day.
Female feedback patterns are more complex. Over the course of the month-long menstrual cycle, female sex hormones exert both positive and negative feedback on GnRH, FSH, and LH.
When circulating levels of the female sex hormones estrogen and progesterone are low, rising follicle stimulating hormone levels trigger egg maturation and estrogen production. Rising estrogen levels induce luteinizing hormone levels to rise. As the levels of female sex hormones rise, they exert negative feedback on FSH secretion, limiting the number of eggs that mature in a month, but positive feedback on LH, eventually producing the LH surge that triggers ovulation. After ovulation, high serum levels of sex hormones again exert negative feedback on GnRH, FSH, and LH which in turn reduces ovarian activity. Levels of female sex hormones therefore decrease, allowing the cycle to start over again.
Many other hormones are not regulated by the pituitary gland, but are released by specific tissues in response to physiological changes. The brain contains receptors for many of these hormones but, unlike pituitary hormones, it does not directly regulate their secretion. Instead, when these hormones bind to receptors on neurons, they modify the output of neural circuits, producing behavioral changes that have homeostatic effects. One example of this is a pair of hormones called leptin and ghrelin.
Leptin and ghrelin change eating behavior by regulating food intake and energy balance. Both hormones affect hunger, and both are released in response to changes in an animal’s internal energy stores. However, they have different effects on the circuits they regulate. Ghrelin keeps the body fed. Released by the wall of the gastrointestinal tract when the stomach is empty, ghrelin activates hunger circuits in the hypothalamus that drive a search for food. Once the stomach is full, ghrelin production stops, reducing the desire to eat. In contrast, leptin helps maintain body weight within a set range. Leptin is produced by fat cells and is released when fat stores are large. When it binds to neurons in the hypothalamus, leptin suppresses the activity of hunger circuits and reduces the desire to eat. As fat stores are used up, leptin levels decline, driving behavior that makes an animal eat more often and replenish its fat stores.
STRESS
Your body reacts in stereotyped ways when you feel threatened. You breathe faster, your heartbeat speeds up, your muscles tense and prepare for action. These reactions may have helped our ancestors run from predators, but any stressful situation — arguing with your parents, a blind date, a looming deadline at work, abdominal cramps, discovering your apartment was robbed, trying karaoke for the first time — has the potential to set them off. Scientists call this reaction the stress response, and your body turns it on to some degree in response to any external or internal threat to homeostasis.
The Stress Response
The stress response weaves together three of the brain’s parallel communication systems, coordinating the activity of voluntary and involuntary nervous systems, muscles, and metabolism to achieve one defensive goal.
Messages sent to muscles through the somatic (voluntary) nervous system prime the body to fight or run from danger (the fight-or-flight response). Messages sent through the autonomic (involuntary) nervous system redirect nutrients and oxygen to those muscles. The sympathetic branch tells the adrenal medulla to release the hormone epinephrine (also called adrenaline), which makes the heart pump faster and relaxes the arterial walls that supply muscles with blood so they can respond more quickly. At the same time, the autonomic system’s parasympathetic branch restricts blood flow to other organs including the skin, gonads, digestive tract, and kidneys. Finally, a cascade of neuroendocrine hormones originating in the hypothalamus and anterior pituitary circulates in the bloodstream, affecting processes like metabolic rate and sexual function, and telling the adrenal cortex to release glucocorticoid hormones — like cortisol — into the blood.
Glucocorticoid hormones bind to many body tissues and produce widespread effects that prepare the body to respond to potential threat. These hormones stimulate the production and release of sugar from storage sites such as the liver, making energy available to muscles. They also bind to brain areas that ramp up attention and learning. And they help inhibit nonessential functions like growth and immune responses until the crisis ends.
It’s easy to imagine how (and why) these physiological changes make your body alert and ready for action. But when it comes to stress, your body can’t tell the difference between the danger of facing down a bull elephant and the frustration of being stuck in traffic. When stress is chronic, whatever its cause, your adrenal glands keep pumping out epinephrine and glucocorticoids. Many animal and human studies have shown that long-term exposure to these hormones can be detrimental.
Chronic stress can also have specific negative effects on brain tissue and function.
Chronic Stress
 Overexposure to glucocorticoids can damage a wide range of physiological systems. It can cause muscles to atrophy, push the body to store energy as fat, and keep blood sugar abnormally high — all of these can worsen the symptoms of diabetes. Overexposure to glucocorticoids also contributes to the development of hypertension (high blood pressure) and atherosclerosis (hardening of the arteries), increasing the risk of heart attacks. Because the hormones inhibit immune system function, they also reduce resistance to infection and inflammation, sometimes pushing the immune system to attack the body’s own tissues.
Overexposure to glucocorticoids can damage a wide range of physiological systems. It can cause muscles to atrophy, push the body to store energy as fat, and keep blood sugar abnormally high — all of these can worsen the symptoms of diabetes. Overexposure to glucocorticoids also contributes to the development of hypertension (high blood pressure) and atherosclerosis (hardening of the arteries), increasing the risk of heart attacks. Because the hormones inhibit immune system function, they also reduce resistance to infection and inflammation, sometimes pushing the immune system to attack the body’s own tissues.
Chronic stress can also have specific negative effects on brain tissue and function. Persistently high levels of glucocorticoids inhibit neuron growth inside the hippocampus, impairing the normal processes of memory formation and recall. Stress hormones can also suppress neural pathways that are normally active in decision-making and cognition, and speed the deterioration in brain function caused by aging. They may worsen the damage caused by a stroke. And they can lead to sleep disorders — cortisol is also an important wakeful signal in the brain, so the high cortisol levels due to chronic stress may delay sleep. Stress-induced insomnia can then start a vicious cycle, as the stress of sleep deprivation leads to the release of even more glucocorticoids.
The effects of chronic stress may even extend beyond a single individual, because glucocorticoids play important roles in brain development. If a pregnant woman suffers from chronic stress, the elevated stress hormones can cross the placenta and shift the developmental trajectory of her fetus. Glucocorticoids are transcription factors, which can bind to DNA and modify which genes will be expressed as proteins. Studies with animal models have shown that mothers with high blood levels of glucocorticoids during pregnancy often have babies with lower birth weights, developmental delays, and more sensitive stress responses throughout their lives.
Because metabolic stressors such as starvation induce high glucocorticoid levels, it’s been suggested that these hormones might help prepare the fetus for the environment it will be born into. Tough, stressful environments push fetuses to develop stress-sensitive “thrifty” metabolisms that store fat easily. Unfortunately, these stress-sensitive metabolisms increase a person’s risk of developing chronic metabolic diseases like obesity or diabetes, especially if they subsequently grow up in lower-stress environments with plentiful food.
The effects of stress can even be passed to subsequent generations by epigenetic mechanisms. Chronic stress can change the markers on DNA molecules that indicate which of the genes in a cell are expressed and which are silenced. Some animal studies indicate that when changes in markers occur in cells that develop into eggs or sperm, these changes can be passed on and expressed in the animal’s offspring. Further research might reveal whether chronic stress has similar effects in humans, and whether inheriting silenced or activated genes contributes to family histories of cancer, obesity, cardiovascular, psychiatric, or neurodevelopmental disease. 
 Almost every cell in your body has an internal clock that tells it when to become active, when to rest, and when to divide. These clocks broker changes in many of the body’s physiological systems over a 24-hour, or circadian, period. For example, the clocks cause faster pulses of peristaltic waves in your gut during the day and make your blood pressure dip at night. But because these clocks are deep inside your body and cannot detect daylight, none of them can tell time on its own. Instead, daily rhythms are coordinated by the suprachiasmatic nucleus (SCN), a tiny group of neurons in the hypothalamus.
Almost every cell in your body has an internal clock that tells it when to become active, when to rest, and when to divide. These clocks broker changes in many of the body’s physiological systems over a 24-hour, or circadian, period. For example, the clocks cause faster pulses of peristaltic waves in your gut during the day and make your blood pressure dip at night. But because these clocks are deep inside your body and cannot detect daylight, none of them can tell time on its own. Instead, daily rhythms are coordinated by the suprachiasmatic nucleus (SCN), a tiny group of neurons in the hypothalamus.


