Neurons develop through delicate and carefully choreographed processes that take place while an embryo grows. Signaling molecules “turn on” certain genes and “turn off” others, initiating the formation of immature nerve cells. During the next stage — cell division, also called proliferation — the pool of early-stage brain cells increases by billions. Finally, during migration, these newly formed neurons travel to their final destinations. The nervous system formed by these processes is active throughout life, making new connections and fine-tuning the way messages are sent and received. In this chapter, you will learn about the amazing early development of your ever-changing nervous system.
THE JOURNEY OF NERVE CELLS
Formation and Induction
During the very early stages of embryonic development, three layers emerge — the ectoderm (outer-most layer), mesoderm (middle layer), and endoderm (inner-most layer). Although the cells in each layer contain identical DNA instructions for development, these layers ultimately give rise to the rich variety of tissue types that make up the human body. The explanation for this diversity lies in signals produced by surrounding tissues. Those signals turn certain genes on and others off, thus inducing the development of specific cell types. Signals from the mesoderm trigger some ectoderm cells to become nerve tissue, a process called neural induction. Subsequent signaling interactions refine the nerve tissue into the basic categories of neurons or glia (support cells), and then into subclasses of each cell type.

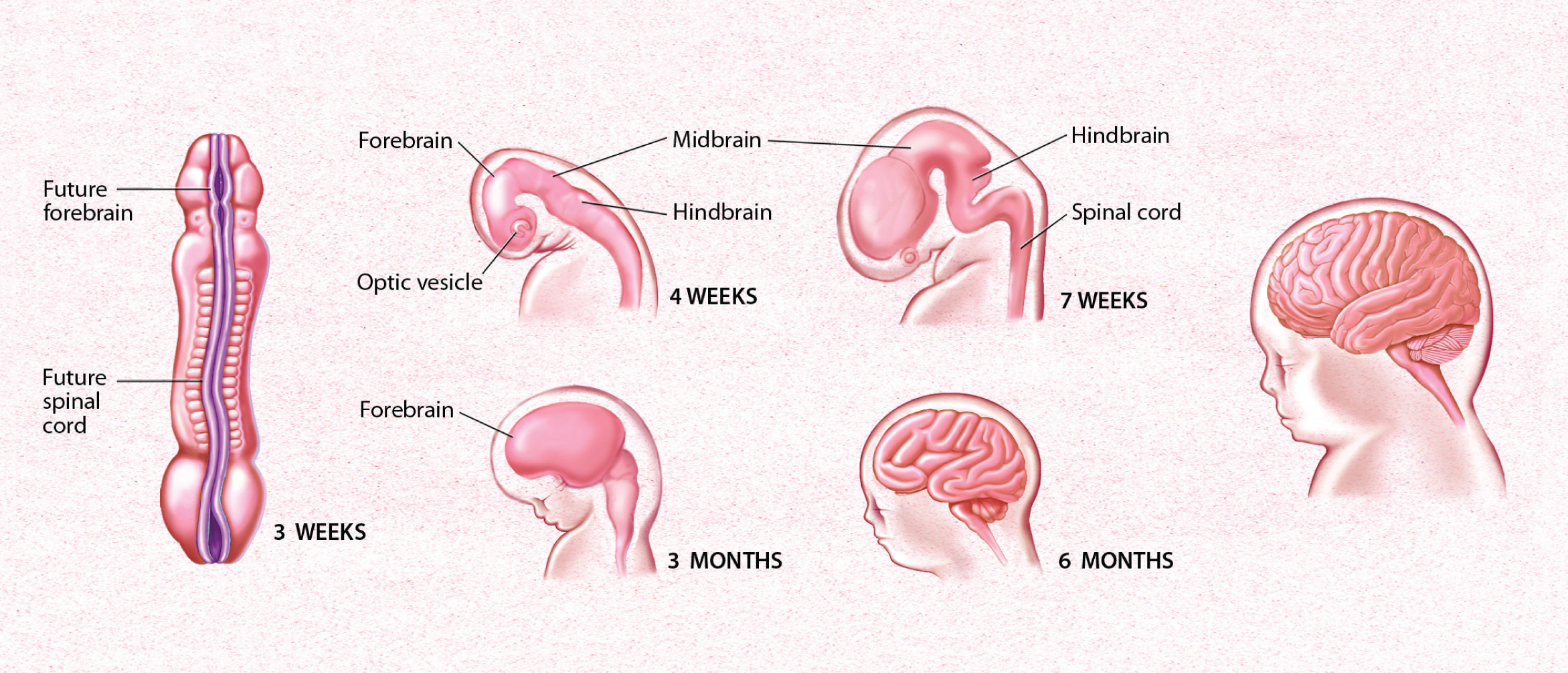
After three week’s gestation, the human brain begins to form. The first stage is the neural tube, pictured at left. By four weeks, the individual sections of the brain can be recognized. In 6 months, the ridges of the brain can be observed.
The fate of a developing cell is largely determined by its proximity to various sources of signaling molecules. The concentration of each type of signaling molecule decreases farther from its source, creating gradients throughout the brain. For example, a particular signaling molecule, called sonic hedgehog, is secreted from mesodermal tissue lying beneath the developing spinal cord. As a result of exposure to this signal, adjacent nerve cells are converted into a specialized class of glia. Cells that are farther away are exposed to lower concentrations of sonic hedgehog, so they become motor neurons that control the movement of muscles. An even lower concentration promotes the formation of interneurons, which don’t relay messages to muscles but to other neurons. Interestingly, the mechanism of this molecular signaling is very similar in species as diverse as flies and humans.
Proliferation
 In the brain, neurons arise from a fairly small pool of neural stem and progenitor cells, special cells that can divide and become a variety of mature cell types. Before achieving their mature cell fate, this pool of cells undergoes a series of divisions — increasing the number of cells that will ultimately form the brain. Early divisions are symmetric — the split results in two identical daughter cells, both able to keep dividing. But as these divisions progress, the cells begin to divide asymmetrically, giving rise to only one daughter cell that keeps proliferating and a second that progresses towards its ultimate cell fate as a neural or glial cell (the exact sequences and ultimate fates vary by species).
In the brain, neurons arise from a fairly small pool of neural stem and progenitor cells, special cells that can divide and become a variety of mature cell types. Before achieving their mature cell fate, this pool of cells undergoes a series of divisions — increasing the number of cells that will ultimately form the brain. Early divisions are symmetric — the split results in two identical daughter cells, both able to keep dividing. But as these divisions progress, the cells begin to divide asymmetrically, giving rise to only one daughter cell that keeps proliferating and a second that progresses towards its ultimate cell fate as a neural or glial cell (the exact sequences and ultimate fates vary by species).
This proliferative process permits rapid growth during early development of the brain, with billions of cells being produced in a matter of weeks. After that series of divisions is complete, only a few neural stem and progenitor cells remain within the brain, and neurogenesis in adulthood is limited to a few regions of the brain, such as those involved with memory.
Scientists have proposed that protein defects causing a premature switch from symmetric to asymmetric divisions may be a cause of microcephaly. This disorder, characterized by a severe reduction in brain size, is associated with serious neurological disabilities and sometimes death in infancy. Similarly, excessive proliferation of brain cells can lead to a disorder called megalencephaly — a brain that is abnormally large and heavy — which is also associated with a variety of neurodevelopmental complications.
Migration
After neural induction and proliferation occur, new neurons journey from the inner surface of the embryonic brain, where they formed, to their long-term locations in the brain. This process is called migration, and it begins three to four weeks after a human baby is conceived. At this time, the ectoderm starts to thicken and build up along the midline of the embryo. As the cells continue to divide, a flat neural plate grows, followed by the formation of parallel ridges, somewhat resembling the creases in a paper airplane, that rise along either side of the midline. These ridges extend from the “head end”, where the future brain will form, along the length of the embryo where the future spinal cord will develop. Within a few days, the ridges fold toward each other and fuse into a hollow neural tube. The head end of the tube thickens into three bulges that form the hindbrain, the midbrain, and the forebrain. Later in the process, at week 7 in humans, the first signs of the eyes and the brain’s hemispheres appear. As new neurons are produced, they move from the neural tube’s ventricular zone, which lies along the inner surface of the tube, toward the border of the marginal zone, or outer surface. After neurons stop dividing, they form an intermediate zone where they gradually accumulate as the brain develops. A variety of guidance cue neurons to migrate to their final destinations.
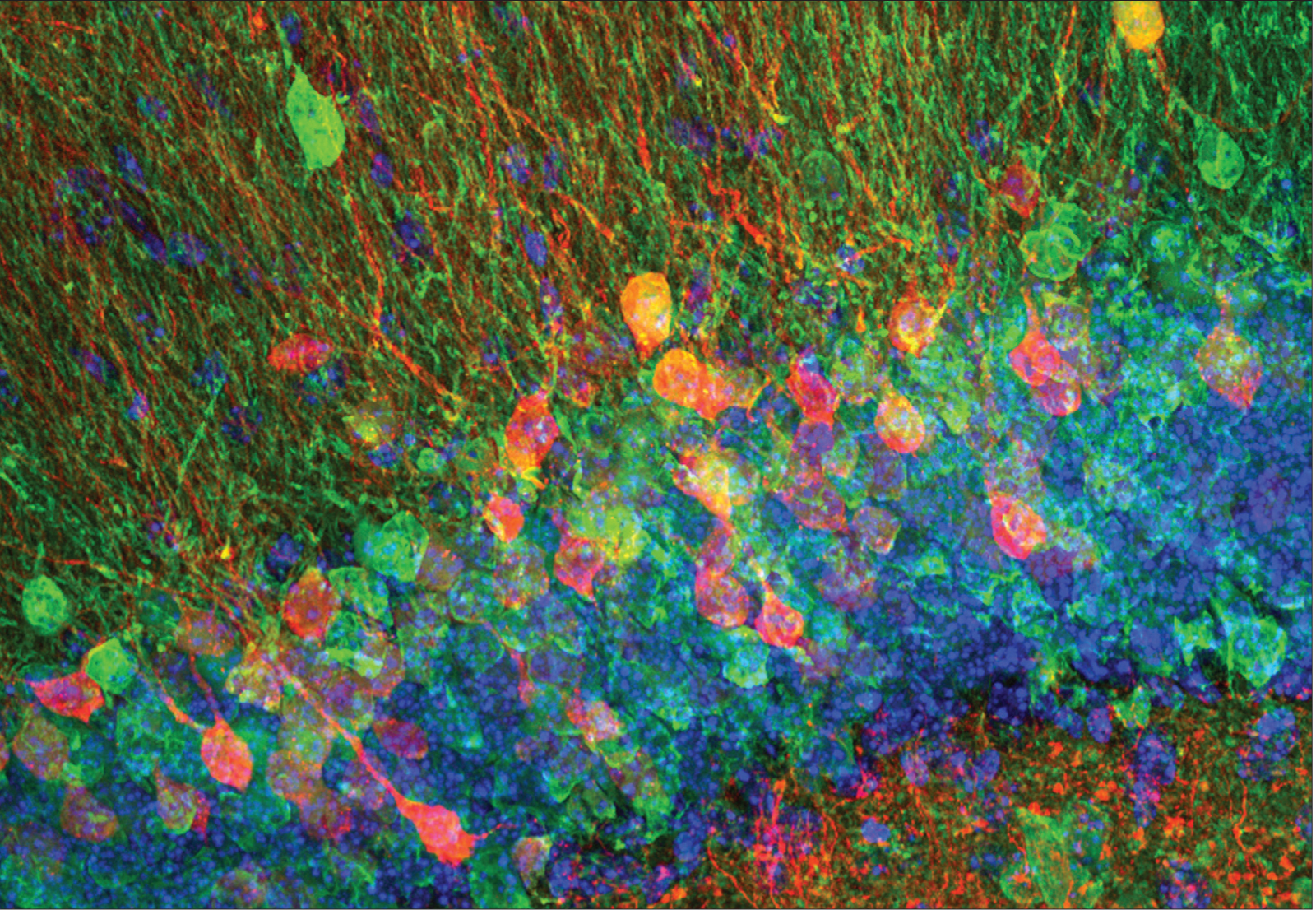
Winkle, et al. The Journal of Neuroscience, 2016
New neurons, shown here in the mouse, are born throughout life in a specific region of the brain’s hippocampus. This region, known as the dentate gyrus, is involved in pattern separation, the ability to discriminate between very similar memories.
The most common guidance mechanism, accounting for about 90 percent of migration in humans, is the radial glia, which project radially from the intermediate zone to the cortex. Neurons use these glia as scaffolding, inching along glial projections until they reach their final destinations. This process of radial migration occurs in an “inside-out” manner; that is, the cells that arrive the earliest (the oldest ones) form the deepest layer of the cortex, whereas the late-arriving (youngest) neurons form the outermost layer. Through a different mechanism, other neurons migrate sideways, or tangentially (rather than radially), moving parallel to the brain’s surface and across the radial cortical columns.
Migration is a finely tuned process that can be influenced by many factors. For example, exposure to alcohol, cocaine, or radiation, can prevent proper migration, resulting in misplacement of cells, which can lead to intellectual disability or epilepsy. Furthermore, mutations in the genes that regulate migration have been shown to cause rare genetic forms of intellectual disability and epilepsy in humans.
Making Connections
 After neurons reach their final locations, they begin making the connections that will determine how particular functions such as vision or hearing can occur. Induction, proliferation, and migration occur internally during fetal development, but the next phases of brain development depend increasingly on external experience. After birth, factors such as watching a mobile spin, listening to a voice, and even proper nutrition influence the connections formed by neurons.
After neurons reach their final locations, they begin making the connections that will determine how particular functions such as vision or hearing can occur. Induction, proliferation, and migration occur internally during fetal development, but the next phases of brain development depend increasingly on external experience. After birth, factors such as watching a mobile spin, listening to a voice, and even proper nutrition influence the connections formed by neurons.
Neurons become interconnected through their short branches called dendrites and long axons — two types of processes that extend from a neuron’s cell body (soma). Axons produce and transmit signals to other neurons, and dendrites receive signals from the axons that contact them. To reach their targets, axons can span distances many times the size of their cell body, many crossing to the opposite side of the brain. The longest human axons are in the periphery, extending from the lower spinal cord all the way to muscles in the toes. Given the distance from spinal cord to toes of a basketball player — a meter or more — such axons might be nearly a million times longer than their diameter!
A developing axon grows by the extension of its growth cone, an enlargement at the tip of the axon that actively explores the environment to seek out its precise destination. A growth cone is guided to that final destination by molecular cues in its environment. Some of these molecules stud the surfaces of cells, while others are secreted into areas near the growth cone. Receptors on the growth cone enable its responses to these environmental cues. Binding of environmental molecules tells the growth cone whether to move forward, stop, recoil, or change direction. Attractive cues lay a path growth cones follow, while repellent molecules funnel growth cones through precise corridors. Signaling molecules include families of proteins with names such as netrin, semaphorin, and ephrin.
One truly remarkable finding is that most of these proteins are common to many organisms — worms, insects, and mammals including humans. Each family of proteins is smaller in flies or worms than in mice or people, but its functions are very similar. As a result, simpler animals are highly useful experimental models for gaining knowledge that directly applies to humans. For example, netrin was first discovered in a worm, where it was found to guide neurons around the worm’s “nerve ring.” Later, vertebrate netrins were found to guide axons around the mammalian spinal cord. When receptors for netrins were then discovered in worms, this knowledge proved invaluable in finding the corresponding, and related, receptors in humans.
Synapse Formation
 Once axons reach their targets, a specialized connection called a synapse begins to form. At the synapse, only a tiny space separates the signaling portion of the axon from the receiving portion of the dendrite. Electrical signals that travel down the axon trigger the release of chemical messages called neurotransmitters, which diffuse across this space and are received by receptors on the target dendrite. Such chemical cues can either promote or hinder the generation of a new electrical signal in the receiving neuron. The combined effects of such cues from thousands of synapses ultimately determine how a receiving neuron responds. A human brain contains trillions of these synapses, which gives rise to the brain’s astounding capacity for information processing.
Once axons reach their targets, a specialized connection called a synapse begins to form. At the synapse, only a tiny space separates the signaling portion of the axon from the receiving portion of the dendrite. Electrical signals that travel down the axon trigger the release of chemical messages called neurotransmitters, which diffuse across this space and are received by receptors on the target dendrite. Such chemical cues can either promote or hinder the generation of a new electrical signal in the receiving neuron. The combined effects of such cues from thousands of synapses ultimately determine how a receiving neuron responds. A human brain contains trillions of these synapses, which gives rise to the brain’s astounding capacity for information processing.
A human brain contains trillions of these synapses, which gives rise to the brain’s astounding capacity for information processing.
For this processing to occur properly, the formation of synaptic connections must be highly specific. Some specificity is the result of the mechanisms that guide each axon to its proper target. Additional molecules mediate target recognition when the axon reaches the proper location. Dendrites are also actively involved in initiating contact with axons, and both sides produce proteins that span the space between them and anchor the synapse together.
Once initial contact is established, a synapse continues to differentiate. On the presynaptic side, the tiny axon terminal that contacts the dendrite becomes specialized for releasing neurotransmitters, stocking itself with neurotransmitter packets, and proteins that enable those packets to be held in place and then released. On the dendritic — or postsynaptic — side, receptors that respond to those neurotransmitters begin to dot the membrane. Both processes ensure that a synapse can transmit signals quickly and effectively.
New evidence has implicated a third important player in the proper formation of a synapse. Astrocytes are a type of glial cell in the brain previously thought to simply provide scaffolding and passive support to neurons. They are now known to exert their own influence on synaptic development and function. Many synapses in the brain are contacted by astrocytes, and studies in rodents have found that a single astrocyte can contact thousands of synapses across multiple neurons. The importance of astrocytes in synapse formation is also shown in other studies. Some neurons form only a few synapses when developing in a culture dish from which astrocytes are absent, and recent research has discovered that molecules secreted by astrocytes regulate aspects of synaptic development.
Scientists are learning that molecules from multiple sources work together to promote proper synapse formation. It is now thought that defects in such molecules could contribute to disorders such as autism. In addition, the loss of certain other molecules might underlie the degradation of synapses that occurs during aging.
An array of signals determines which type of neurotransmitter a neuron will use to communicate. For some cells, such as motor neurons, the type of neurotransmitter is fixed (acetylcholine), but for other neurons, it is not. Scientists have found that when certain immature neurons are maintained in a culture dish with no other cell types, they produce the neurotransmitter norepinephrine. In contrast, when the same neurons are cultured with specific cells, such as cardiac tissue, they produce the neurotransmitter acetylcholine. Just as genetic and environmental signals can modulate the development of specialized cells, a similar process leads to production of specific neurotransmitters. Many researchers believe that the signal to engage the gene, and therefore the final determination of the chemical messenger a neuron will produce, is influenced by factors that come from the location of the synapse itself.
Insulation that covers wires preserves the strength of electrical signals that travel through them. The myelin sheath that covers axons serves a similar function. Myelination, the fatty wrapping of axons by extensions of glia, increases — by as much as 100 times — the speed at which signals can travel along axons. This increase is a function of how the sheath is wrapped, with somewhat regularly spaced gaps called nodes of Ranvier interrupting the sheath. The alternating pattern of insulation and nodes allows electrical signals to move down an axon faster, jumping from one node to the next. This phenomenon, called saltatory conduction (“saltatory” means “leaping”), is responsible for more rapid transmission of electrical signals. Formation of myelin occurs throughout the lifespan.
Paring Back
 After its initial growth, the neural network is pared back, creating a more efficient system. In fact, only about half the neurons generated during development survive to function in an adult. Entire populations of neurons are removed through apoptosis, a process of programmed cell death initiated in the cells. Apoptosis is activated if a neuron fails to receive enough life-sustaining chemical signals called trophic factors, which are produced in limited quantities by target tissues. Each type of trophic factor supports the survival of a distinct group of neurons. For example, nerve growth factor is important for the survival of sensory neurons. It has recently become clear that apoptosis is maintained into adulthood but constantly held in check. Based on this, researchers have found that injuries and some neurodegenerative diseases kill neurons not by directly inflicting damage but by activating the cells’ own death programs. This discovery — and its implication that death need not follow insult — have led to new avenues for therapy.
After its initial growth, the neural network is pared back, creating a more efficient system. In fact, only about half the neurons generated during development survive to function in an adult. Entire populations of neurons are removed through apoptosis, a process of programmed cell death initiated in the cells. Apoptosis is activated if a neuron fails to receive enough life-sustaining chemical signals called trophic factors, which are produced in limited quantities by target tissues. Each type of trophic factor supports the survival of a distinct group of neurons. For example, nerve growth factor is important for the survival of sensory neurons. It has recently become clear that apoptosis is maintained into adulthood but constantly held in check. Based on this, researchers have found that injuries and some neurodegenerative diseases kill neurons not by directly inflicting damage but by activating the cells’ own death programs. This discovery — and its implication that death need not follow insult — have led to new avenues for therapy.
Just as too many brain cells develop early on, these cells initially form an excessive number of connections. In primates, for example, neural projections from the two eyes to the brain initially overlap; then, in some portions of the brain, they sort into separate territories devoted to one eye or the other. Furthermore, connections between neurons in a young primate’s cerebral cortex are more numerous and twice as concentrated as in an adult primate. The pruning of these excess connections is heavily dependent on the relative activity of each connection. Connections that are active and generating electrical currents survive, while those with relatively little activity are lost. Astrocytes and other glia also play an important role in this process. For example, astrocytes are known to aid the formation of eye-specific connections by engulfing and eliminating unnecessary synapses. Thus, at least to some extent, the circuits of the adult brain are formed by pruning away incorrect connections to leave only the correct ones. 