 Our understanding of how humans learn and remember is far from complete, but researchers are uncovering intriguing new details about the mechanisms, limits, and architecture of memory formation.
Our understanding of how humans learn and remember is far from complete, but researchers are uncovering intriguing new details about the mechanisms, limits, and architecture of memory formation.A patient known for most of five decades only by his initials, H.M., led to one of the most significant turning points in 20th century brain science: the understanding that complex functions such as learning and memory are tied to distinct biological processes and regions of the brain.
Following a childhood blow to the head, Henry Molaison developed severe seizures. Eighteen years later, still experiencing debilitating symptoms, he underwent an experimental procedure that removed sections of his medial temporal lobes — including most of his two hippocampi. The seizures abated, but Molaison was left with permanent amnesia. He could remember scenes from his childhood, some facts about his parents, and historical events that occurred before his surgery, but was unable to form new conscious memories.
For example, if Molaison met someone who then left the room, within minutes he had no recollection of the person or their meeting. He experienced every aspect of his daily life — eating a meal, taking a walk — as a first. Yet his intellect, personality, and perception were intact, and he was able to acquire new motor skills. Over time, he became more proficient at tasks such as tracing patterns while watching his hand movements in a mirror, despite the fact that he could never recall performing the task before.
Studied by neuroscientists for 50 years, until his death in 2008 at age 82, Molaison’s intact abilities as well as his impairments provided evidence for the roles of the hippocampus and parahippocampal region in converting memories from short-term to long-term, paving the way for further exploration of brain networks encoding conscious and unconscious memories.
LEARNING AND MEMORY
 Our understanding of how humans learn and remember is far from complete, but researchers are uncovering intriguing new details about the mechanisms, limits, and architecture of memory formation.
Our understanding of how humans learn and remember is far from complete, but researchers are uncovering intriguing new details about the mechanisms, limits, and architecture of memory formation.
Thanks in part to H.M., scientists now know that the medial temporal lobe, which includes the hippocampus and parahippocampal regions, works with other regions of the cerebral cortex, the brain’s outermost layer, to form, organize, consolidate, and retrieve memories. The four major lobes of the cerebral cortex — frontal, parietal, temporal, and occipital — process sensory information such as smell, taste, sight, and sound. Associative regions in the cortex integrate these sensory inputs, enabling us to understand our environment and encode memories.
Declarative Memory
Declarative memory is memory for facts, data, and events. Such conscious (explicit) memories are called declarative memories because you can consciously recall and describe the information. Declarative memories can be semantic or episodic. Semantic memories consist of the cultural knowledge, ideas, and concepts you’ve accumulated about the world — for example, names of state capitals, word definitions, how to add and subtract, or dates of historical events and their meaning. This type of memory involves cortical regions well beyond the hippocampus. Episodic memories are unique representations of your personal experiences. For example, mentally recalling the sights, sounds, time, space, and emotions associated with an experience involves episodic memory.
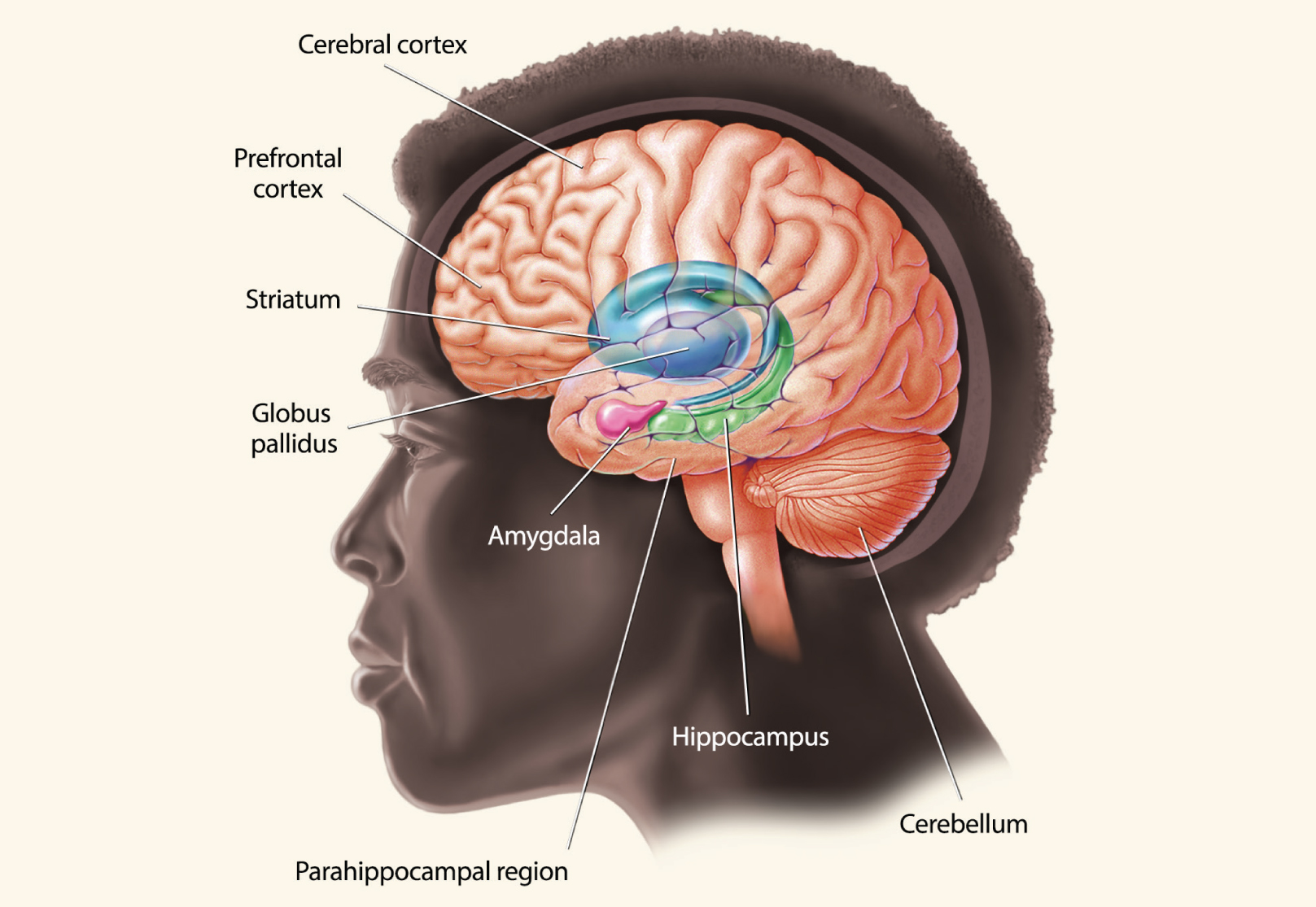
There are several different kinds of memory, and they are processed by different areas of the brain. The hippocampus, parahippocampal region, and areas of the cerebral cortex work in tandem to produce memories of facts and events. Other kinds of memories, such as emotional or behavioral memories, are handled by other parts of the brain, including the amygdala, striatum, and cerebellum.
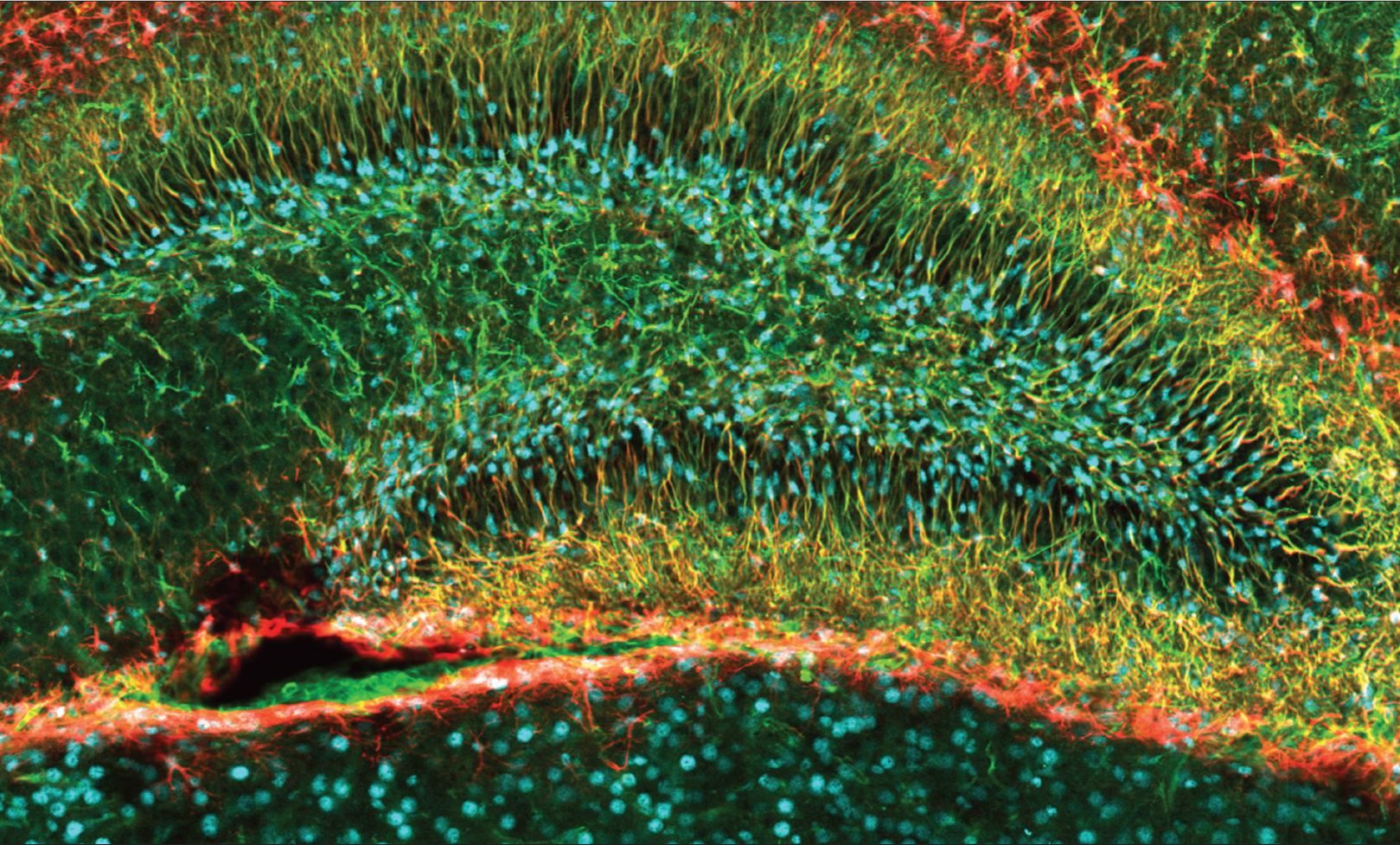
Noguchi, et al. The Journal of Neuroscience, 2016.
The dentate gyrus, a portion of the hippocampus responsible for memories of events, is one of the few areas of the adult brain where neurogenesis takes place. This image of a mouse dentate gyrus shows newborn cells, labeled in blue, along with the support cells called glia, labeled in red, that will help them migrate to their final destinations. Some of these new cells will mature to become different types of neurons in the dentate gyrus, where they will play important roles in learning and memory.
Interestingly, the emotional significance attached to memories of events and experiences is mediated by the amygdala. A paired structure consisting of two almond-shaped regions (amygdala comes from the Greek word for almond), the amygdala modulates “fight-or-flight” responses linked to survival. The parahippocampal region also aids the hippocampus in encoding the “what” of episodic memories, rather than the “where” or “when.”
The type of memory described so far is the long-term form of declarative memory. Such memories are stored throughout a broad network of cortical areas. H.M. was able to retrieve his previous long-term memories, but not able to form new ones.
The brain seems to have unlimited capacity for long-term memories, but short-term memories are limited to small sums of data for a limited time.
In contrast, working memory is a temporary type of declarative memory, a form of short-term memory that lets you hold a phone number, a sum, a visual image, or other data point needed in the present and immediate future. While the brain seems to possess unlimited capacity for long-term memories, short-term memories are limited to relatively small amounts of data for a limited amount of time. These data are accessible while they’re being processed and manipulated but, unless transferred to long-term memory, they decay after only a few seconds and can no longer be retrieved.
Some aspects of working memory are coordinated by the prefrontal cortex (PFC), the “brain’s executive,” which also controls attention, decision-making, and long-term planning. Specific areas of the PFC monitor information from long-term memory as well as coordinating working memory from multiple brain regions. Brain imaging studies demonstrate the PFC is particularly active when people concentrate on keeping something like a phone number in mind. Animal studies suggest that neurons in the PFC fire in spurts, keeping information active or “online” in working memory. H.M. did not lose this type of memory.
Spatial memory is another facet of declarative memory. This was identified in studies showing that discrete areas, and even individual neurons within the brain, are dedicated to processing specific types of information. For example, navigational memories involved in creating mental maps are tied to specific types of neurons. So-called “place cells” in the hippocampus light up as you move through a familiar house or room, or as a rat navigates a known maze. Studies have also shown that mice navigating a maze display specific sequences of neuronal activity devoted to right or left turns. These patterns become increasingly distinct as the animals learn the maze. Studies have even shown that learning complex navigational routes causes changes in the hippocampus.
“Grid cells,” don’t represent particular locations. Located in the entorhinal cortex, an area near the hippocampus, they represent coordinates that allow the brain to track your position in space when landmarks or external cues are absent.
Nondeclarative Memory
Nondeclarative memory — also known as implicit or procedural memory — is stored and retrieved without conscious effort. You use this type of memory when you perform learned motor skills like speaking or riding a bike. H.M. did not lose this type of memory, as evident in his ability to acquire new motor skills, even though he couldn’t remember doing them before.
The fact that H.M. (and other people with amnesia) show deficits in some types of memory but not others indicates that different types of memories are encoded in separate, but interacting, regions of the brain. Motor skill learning, for example, involves many areas of the brain, but three are especially important: the basal ganglia — the “habit center” of the brain — the prefrontal cortex, and the cerebellum, an area at the back of the brain involved in motor control and coordination.
Storing Memories in Your Synapses
 Your brain is able to form memories and rewire itself in response to experience because circuits in your brain change at synapses — the tiny gaps across which neurons communicate via chemical and electrical signals. The ability of synapses to remodel themselves is called synaptic plasticity. Encoding a new long-term memory involves persistent changes in the number and shape of synapses, as well as the amount of neurotransmitter released and the number of receptors on the postsynaptic membrane.
Your brain is able to form memories and rewire itself in response to experience because circuits in your brain change at synapses — the tiny gaps across which neurons communicate via chemical and electrical signals. The ability of synapses to remodel themselves is called synaptic plasticity. Encoding a new long-term memory involves persistent changes in the number and shape of synapses, as well as the amount of neurotransmitter released and the number of receptors on the postsynaptic membrane.
In transmitting information from one neuron to another, a presynaptic (sending) neuron transforms an electrical signal into the release of chemical messengers called neurotransmitters that diffuse across the synaptic gap to the postsynaptic (receiving) neuron. The membrane of the postsynaptic neuron contains proteins called receptors that interact with neurotransmitters. Upon binding the neurotransmitters, the receptors unleash a cascade of molecular events that convert the message back into an electrical signal. The receptors then release the neurotransmitters, which are recycled back into the presynaptic terminal or broken down enzymatically, allowing postsynaptic receptors to receive new signals from the presynaptic neuron.
Scientists have learned a great deal about the ways presynaptic and postsynaptic neurons remodel themselves. The sea slug, Aplysia californica, was an important animal model for the first neuroscientists studying synaptic plasticity because its nerve cells are relatively few and easy to observe. Researchers identified chemical and structural changes in relevant nerve cells of Aplysia that correlated with simple forms of learning and memory. Studies in genetically modified mice have revealed that alterations in gene expression facilitate long-term changes in synaptic structure. Genes governing a type of glutamate receptor — N-methyl-d-aspartate (NMDA) receptors — and a molecule called cAMP-response element binding protein (CREB) are especially important in the formation of long-term memories.
Two opposing but equal processes are key for synaptic plasticity: long-term potentiation (LTP) and long-term depression (LTD). LTP is a long-lasting increase in synaptic strength, which occurs in many brain regions but especially in the hippocampus. LTD, conversely, decreases a synapse’s effectiveness. Experience physically changes our brains through LTP, shown in numerous animal and human studies to be essential for long-term memory consolidation.
While LTP has been identified throughout the brain, it has been studied extensively in the hippocampus, the brain region associated with encoding new memories. The precise mechanism of LTP varies depending on the type of neurons, but, in general, it involves an increase in the number of glutamate receptors on the postsynaptic neuron. Glutamate is the most prevalent neurotransmitter in the mammalian nervous system, and it binds to several different kinds of receptors. The NMDA and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) classes of glutamate receptors are ion channels. Upon binding glutamate, they permit calcium and sodium ions, respectively, to flow into the cell. Increasing the number of receptors on the postsynaptic cell strengthens a synapse by allowing the entry of more electrically conductive ions.
Calcium ions also function as second messengers — signaling molecules that set off a chain of molecular events within cells. LTP boosts the concentration of calcium ions inside a postsynaptic cell, while LTD increases it to a lesser degree. The differing concentrations of calcium activate different enzymes: kinase proteins in the case of LTP, or phosphatases for LTD. These enzymes modify the synapse, making it more or less efficient at relaying nerve impulses.
In LTP, a series of molecular events stabilizes the synaptic changes: The increase in calcium ions within the post-synaptic cell activates cyclic adenosine monophosphate (cAMP) molecules. This, in turn, activates several kinds of enzymes, some of which increase the number of synaptic receptors, making the synapse more sensitive to neurotransmitters. In addition, continued stimulation through repetitive experience activates CREB. CREB acts in the nucleus of the neuron to switch on a series of genes, many of which direct protein synthesis. Among the many proteins produced are neurotrophins, which stimulate the growth of the synapse and structural elements, stabilizing increased sensitivity to neurotransmitters.
The preceding molecular cascade is essential for memories to become long-term. The prevailing view is that declarative memories are encoded in the hippocampus, then transferred to the frontal lobes for long-term storage and consolidation. Research suggests that, over time, the hippocampus becomes less important for retrieving older memories as the frontal cortex assumes that task.
As researchers gain new insights into the molecular mechanisms underlying memory, pharmaceutical and technological advances may enable artificial manipulation of synaptic plasticity. New treatments could be developed for synapse-related neurological disorders — such as eradication of harmful memories tied to post-traumatic stress disorder (PTSD) — or for boosting our ability to learn and remember.
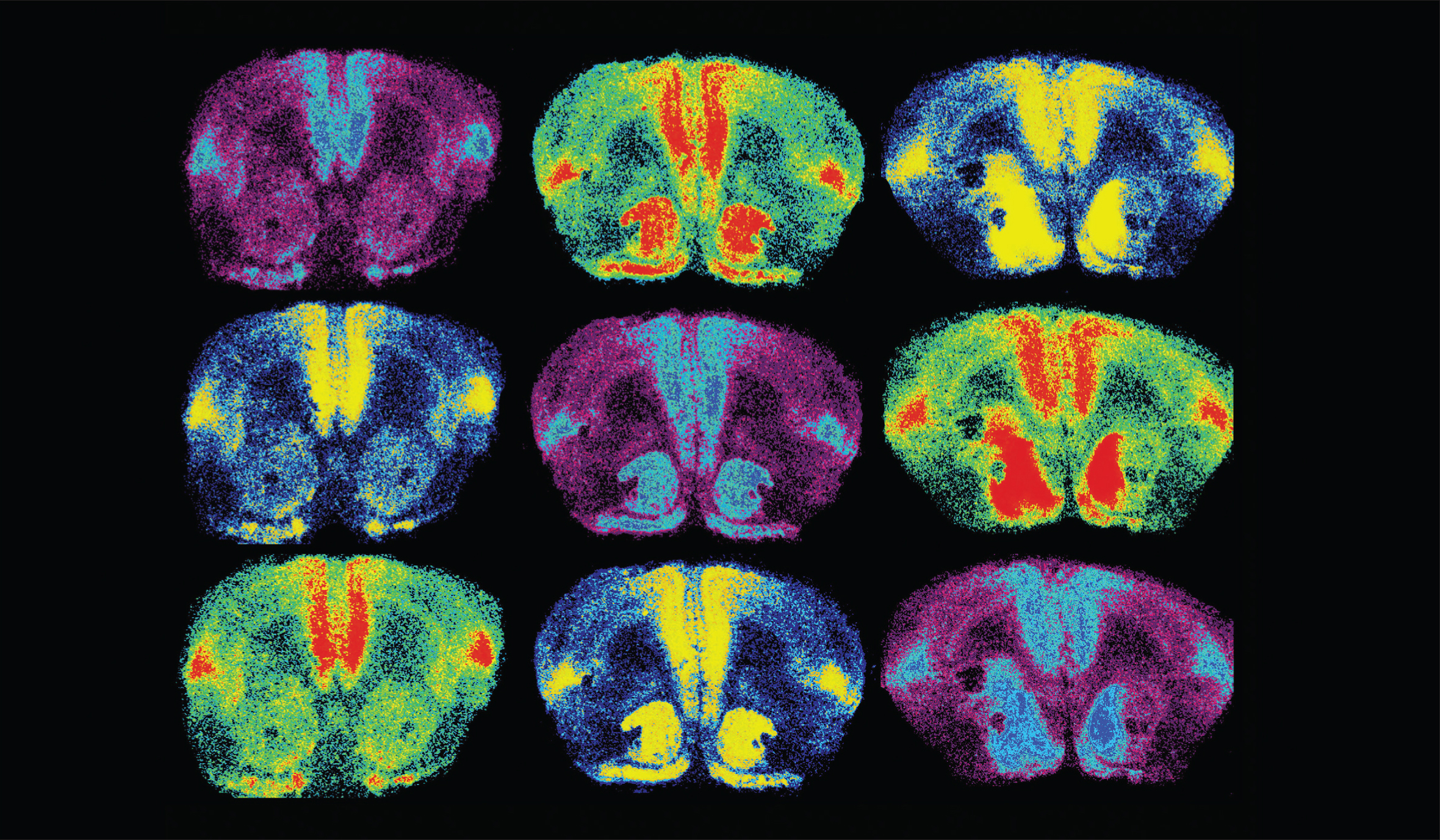
Ross, et al. The Journal of Neuroscience, 2009.
Oxytocin is a brain chemical closely associated with love. In order to study something as unique as love, researchers look at the brains of prairie voles, which mate for life. In this image, oxytocin receptors are labeled in light blue, red, and yellow. When researchers increased oxytocin receptor levels in the brain (right column), they found female voles formed partner preferences faster.
EMOTIONS
 In emotional memory, considered another type of nondeclarative memory, learned emotional responses become attached to stimuli over time after repeated exposure. In the 1970s, anthropologist Paul Ekman identified what he called the six basic emotions: anger, fear, surprise, disgust, joy, and sadness. While scientists have since disputed the exact number and attributes of human emotions, whether emotions are consistent across cultures, or even how to define an emotion, their research has linked some neural circuits to physiological responses that help us survive, interact, set goals, and initiate actions.
In emotional memory, considered another type of nondeclarative memory, learned emotional responses become attached to stimuli over time after repeated exposure. In the 1970s, anthropologist Paul Ekman identified what he called the six basic emotions: anger, fear, surprise, disgust, joy, and sadness. While scientists have since disputed the exact number and attributes of human emotions, whether emotions are consistent across cultures, or even how to define an emotion, their research has linked some neural circuits to physiological responses that help us survive, interact, set goals, and initiate actions.
Anatomy of Emotion
The brain structures most closely linked with emotions are the amygdala, the insula or insular cortex, and the periaqueductal gray, located in the midbrain. Neurons from the prefrontal cortex, the amygdala, and the insular cortex project to the periaqueductal gray, which in turn has reciprocal connections with the central nucleus of the amygdala and projections to the thalamus, hypothalamus, brainstem, and deep layers of the spinal cord.
The amygdala integrates emotions, emotional behavior, and motivation. It interprets fear, helps distinguish friends from foes, and identifies social rewards and how to attain them. One very familiar type of learning is dependent on the amygdala: classical conditioning, which associates a stimulus with reward or punishment.
Through the insula, you experience disgust — a strong negative reaction to an unpleasant odor, for instance — that might protect you from ingesting poison or spoiled food. The insula has also been implicated in feeling and anticipating pain, although its exact function in this arena is not well understood. The insula is believed to take in system-wide inputs and generate subjective feelings about them; thus linking feelings, internal physiological states, social emotions, and conscious actions.
The periaqueductal gray, located in a region where incoming sensory information is acted on by higher brain centers, has been tied to pain perception as well as stress responses including defensive and reproductive behaviors, maternal attachment, and anxiety. Receptors for pain-reducing compounds such as morphine and oxycodone are clustered in the periaqueductal gray.
Motivation: Affective Decision-Making
Human actions are driven by necessities — food, sleep, sex, avoidance of pain — and by rewards, but our responses and actions are not always logical. While little is known about exactly how the brain transforms feelings into decisions, researchers have developed theoretical models about decision-making. Affective decision-making involves choices under risky and uncertain conditions. An active area of neuroscience research is investigating how the brain balances reward and risk, and how emotional state affects this balance.
Emotionally centered decision-making changes with age — possibly because the lateral prefrontal cortex, responsible for self-regulation, matures gradually in adolescents. Teens’ developing brains and high sensitivity to peer acceptance might be related to their increased tolerance for risky behaviors. Older adults might also make more risky decisions, as PFC function diminishes with age.
Motivation: Dopamine and Reward Pathways
Although relatively few neurons in the mammalian central nervous system generate the neurotransmitter dopamine, these dopaminergic neurons influence multiple brain functions including voluntary movement and a variety of behavioral processes such as mood, reward, addiction, stress and memory.
When something is very rewarding, we are more likely to remember it. That is because dopamine influences the synapses in the entire reward pathway — the hippocampus, amydgala, and the prefrontal cortex — to create emotional associations with rewards. And the mesolimbic pathway, sometimes called the “reward pathway,” is a major pathway for dopamine, connecting the mid-brain’s ventral tegmental area (VTA) to the nucleus accumbens. It is involved in cognitive processing of rewards and motivation. Neurons that release dopamine are activated in response to signals that a reward will be given.
Surprisingly, it’s not the reward itself, but the expectation of a reward that most powerfully influences the emotional reaction. Reward learning occurs in response to something unexpected — when the actual reward differs from what was predicted. If a reward is greater than anticipated, dopamine signaling increases. If a reward is less than expected, dopamine signaling decreases. In contrast, a correctly predicted reward does not elicit changes in dopamine signaling, and all remains the same.
Interestingly, recent research shows that dopaminergic responses vary among people. Some people’s brains respond more strongly to rewards than punishments, while others respond more strongly to punishments. The amygdala has been implicated in various aspects of reward learning and motivation. Researchers at Vanderbilt University found that “go-getters” who are more willing to work hard have greater dopamine signaling in the striatum and prefrontal cortex — two areas known to impact motivation and reward.
While the brain’s reward system typically reinforces behaviors associated with rewards and prevents behaviors leading to punishment, aberrant circuitry can lead to inappropriate aggression, a symptom of some neuropsychiatric disorders. For example, the lateral habenula, a major node in the reward circuitry, appears to encode punishment by inhibiting dopamine release, and dysfunction of the lateral habenula has been linked to disorders involving inappropriate aggression. The amygdala has also been associated with negative emotions. Stimulating some areas can trigger rage and aggression, while removing specific sections of the amygdala will make lab animals more docile. Recent studies in lab animals have also suggested that aggression can result from inappropriate activation of the brain’s reward systems in response to violent social stimuli. 